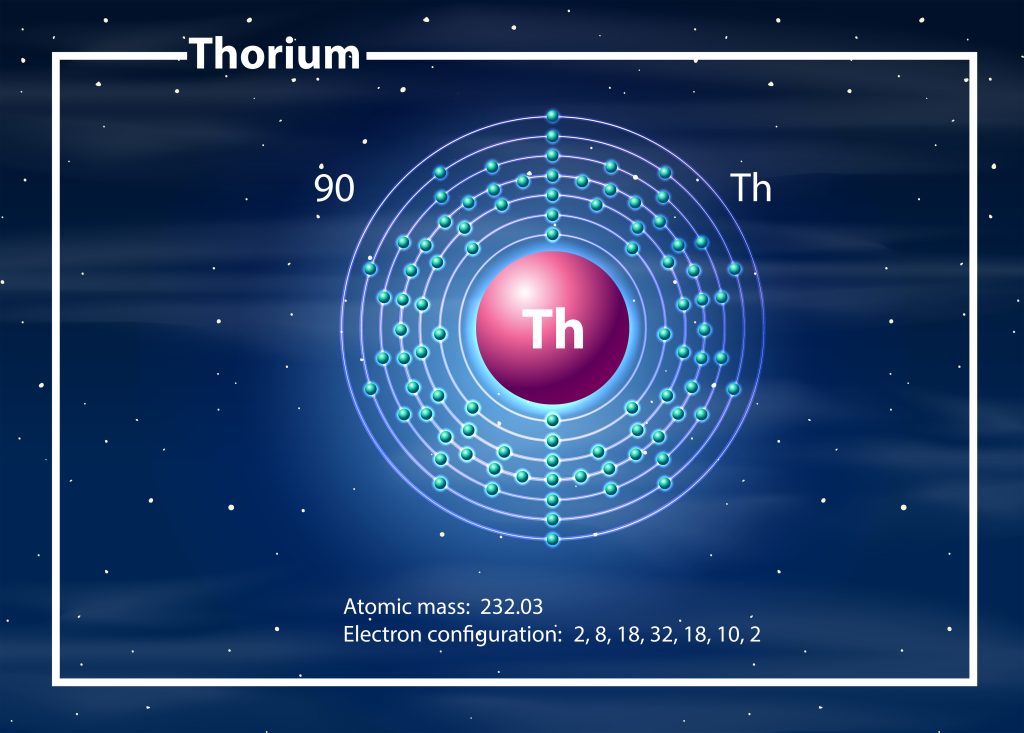
Have you ever wondered how many protons are in thorium? Well, in this article, we’ll answer that question for you. Thorium is an element that has a crucial role in various applications, particularly as a nuclear fuel. To understand its properties and behavior, it’s essential to know its atomic structure, including the number of protons it contains. The most stable isotope of thorium, Thorium-232, has an atomic number of 90, which tells us the number of protons in its nucleus. Alongside protons, it also has neutrons and electrons that contribute to its overall composition. By exploring the nuclear composition of thorium, we can gain valuable insights into its stability and its use in nuclear applications. So, let’s dive in and discover the fascinating world of thorium’s atomic structure.
Background on Atoms
Understanding the background of atoms is essential for grasping the concept of how many protons Thorium has. Atoms are the smallest particles of an element involved in chemical reactions. They consist of a nucleus and orbits, with protons and neutrons in the nucleus and electrons revolving around it. Protons, neutrons, and electrons are the permanent particles located in the atom. A thorium atom contains 90 protons, which are the core particles located in the nucleus. The mass of a proton is approximately 1837 times greater than the mass of an electron. Electrons, on the other hand, are the core particles located in specific orbits around the nucleus. The number of protons and electrons in an element is equal, which means that a thorium atom also contains 90 electrons. Neutrons are charge-neutral particles located in the nucleus of an atom. A thorium atom has 142 neutrons. Thorium-232, the most stable isotope of thorium, is used as a nuclear fuel and has 90 protons, 142 neutrons, and 90 electrons.
Protons in a Thorium Atom
Thorium atom contains 90 protons. Here are some key details about protons in a thorium atom:
- Proton Charge: The actual charge of a proton is +1.602 × 10^-19 coulombs.
- Proton Mass: The mass of a proton is approximately 1837 times greater than the mass of an electron.
- Proton Diameter: The diameter of a proton particle is about 2.4 × 10^-13 cm.
- Proton Location: Protons are permanent core particles located in the nucleus of an atom.
In a thorium atom, the nucleus contains 90 protons. Protons are positively charged particles with a mass that is much greater than that of electrons. They are located in the nucleus, which is the central part of the atom. The charge of a proton is +1.602 × 10^-19 coulombs, and its diameter is about 2.4 × 10^-13 cm. These protons, along with neutrons, make up the majority of the mass of the atom. Understanding the properties and location of protons in a thorium atom is essential for studying its atomic structure and behavior.
Electrons in a Thorium Atom
To understand the composition of a thorium atom, it is important to examine the role of electrons within it. Electrons are permanent core particles located in specific orbits around the nucleus. The mass of an electron is about 1/1836 of the mass of a hydrogen atom, and its charge is -1.609 × 10^-19 coulombs. In a thorium atom, there are 90 electrons, which is equal to the number of protons. The electron arrangement in a thorium atom can be represented by the electron configuration [Rn] 6d2 7s2. This arrangement determines the oxidation behavior of thorium, with thorium primarily exhibiting an oxidation state of +4. The arrangement of electrons in an atom plays a crucial role in its chemical behavior. Moreover, the stability of a thorium atom is determined by the number of protons, neutrons, and electrons. Thorium-232, the most stable isotope of thorium, has 90 protons, 142 neutrons, and 90 electrons. Understanding the electron arrangement in a thorium atom is essential for comprehending its chemical properties and nuclear stability.
| Particle | Mass (kg) | Charge (coulombs) |
|---|---|---|
| Proton | 1.6726 × 10^-27 | +1.602 × 10^-19 |
| Neutron | 1.674 × 10^-27 | 0 |
| Electron | 9.1094 × 10^-31 | -1.609 × 10^-19 |
Neutrons in a Thorium Atom
You can determine the number of neutrons in a thorium atom by subtracting the atomic number from the atomic mass number. Here are some key points about neutrons in a thorium atom:
- Atomic Mass: The atomic mass of thorium is approximately 232.0377 atomic mass units (u).
- Nuclear Stability: Neutrons play a crucial role in maintaining nuclear stability by offsetting the electrical repulsion between protons in the nucleus.
- Nuclear Decay: Thorium isotopes undergo radioactive decay, emitting alpha particles or undergoing beta decay. The number of neutrons in an atom can affect its stability and decay process.
- Chemical Behavior: Neutrons do not significantly affect the chemical behavior of thorium compounds. The chemical properties of thorium are primarily determined by its outermost electrons.
Isotopes of Thorium
To understand the different variations of thorium, it is important to explore the isotopes of this element. Thorium has a total of thirty-two isotopes, with each isotope having the same number of protons but varying numbers of neutrons. These isotopes of thorium play a significant role in nuclear reactions, radioactive decay, and the use of thorium in various applications such as thorium compounds and nuclear power.
To give you a better understanding of the different isotopes of thorium, here is a table showcasing some of the notable isotopes:
| Isotope | Number of Protons | Number of Neutrons | Radioactive Decay |
|---|---|---|---|
| Thorium-228 | 90 | 138 | Alpha Decay |
| Thorium-230 | 90 | 140 | Alpha Decay |
| Thorium-232 | 90 | 142 | Alpha Decay |
| Thorium-234 | 90 | 144 | Alpha Decay |
These isotopes undergo various types of radioactive decay, emitting alpha particles in the process. Thorium-232, the most stable isotope of thorium, is commonly used as a nuclear fuel due to its long half-life of 14 billion years. Understanding the different isotopes of thorium is crucial in harnessing its potential in nuclear power and other applications.
Electron Configuration and Oxidation States
The electron configuration of Thorium is determined by the arrangement of electrons in its atom. Here are some key points about the electron configuration and oxidation states of Thorium:
- Electron Configuration:
- The electron configuration of Thorium is [Rn] 6d2 7s2.
- This means that Thorium has two electrons in the 6d orbital and two electrons in the 7s orbital.
- Oxidation States:
- Thorium primarily exhibits an oxidation state of +4.
- Oxidation states are represented by integers and can be positive, zero, or negative.
- Most elements have more than one possible oxidation state.
- Chemical Behavior:
- The electron configuration of Thorium influences its chemical behavior.
- The outermost electrons determine how Thorium interacts with other elements to form compounds.
- The +4 oxidation state of Thorium allows it to form stable compounds with various elements.
- Nuclear Stability and Radioactive Decay:
- Thorium-232 is the most stable isotope of Thorium.
- It has a half-life of 14 billion years and decays by emitting an alpha particle to form radium-228.
- Unstable isotopes of Thorium undergo radioactive decay through various decay pathways.
Understanding the electron configuration and oxidation states of Thorium is crucial in comprehending its chemical behavior, nuclear stability, and radioactive decay.
Unstable Isotopes and Radioactive Decay
Unstable isotopes of Thorium undergo radioactive decay at varying frequencies. These isotopes have different half-lives and decay through various pathways. Here is a table summarizing the half-life and decay pathways of some unstable isotopes of Thorium:
| Isotope | Half-life | Decay Pathway |
|---|---|---|
| Thorium-227 | 18.68 days | Alpha decay |
| Thorium-228 | 1.9116 years | Alpha decay |
| Thorium-229 | 7917 years | Alpha decay |
| Thorium-230 | 75400 years | Alpha decay |
| Thorium-231 | 25.5 hours | Beta decay |
These decay processes result in the emission of alpha or beta particles, eventually transforming the Thorium isotopes into different elements.
While unstable Thorium isotopes have limited practical applications, Thorium dioxide (thoria) is widely used. It has high melting point properties, reaching 3300 °C (6000 °F), making it suitable for high-temperature applications. Thorium dioxide is used as a refractory material in gas tungsten arc welding electrodes, crucibles, and high-temperature ceramics. Its stability and high melting point make it valuable in industries where extreme conditions are involved.
Thorium Dioxide and Its Properties
When discussing Thorium Dioxide and its properties, it is important to note that this compound is widely used in various applications. Here are some key points about thorium dioxide:
- Uses of thorium dioxide:
- Thorium dioxide is commonly used as a nuclear fuel in reactors due to its ability to produce large amounts of energy.
- It is also utilized in the manufacture of high-temperature ceramics, such as crucibles and refractory materials, because of its exceptional heat resistance.
- Thorium dioxide is employed in the production of gas mantles for portable camping lanterns, as it produces a bright white light when heated.
- Additionally, it is used as a catalyst in various chemical reactions.
- Properties of thorium dioxide:
- Thorium dioxide has a very high melting point of 3300 °C (6000 °F), making it one of the compounds with the highest melting points.
- It is insoluble in water and most acids, making it highly resistant to corrosion.
- Thorium dioxide is a white, crystalline solid that is odorless and non-volatile.
- Comparison of thorium dioxide with other compounds:
- Compared to thorium metal, thorium dioxide is more stable and less reactive.
- Thorium dioxide has a higher melting point compared to many other compounds, including uranium dioxide and plutonium dioxide.
- Safety considerations and environmental impact of thorium dioxide:
- Thorium dioxide is a radioactive material, and exposure to its dust or particles can pose health hazards, including radiation damage.
- Proper safety measures and handling protocols should be followed when working with thorium dioxide to minimize risks.
- The environmental impact of thorium dioxide is a subject of debate, as its long-term effects on ecosystems are still being studied.