You’ve heard of atoms, but what about isotopes? You’re about to dive into the fascinating world of atomic variations. You’ll learn how isotopes are formed, the different types, and their real-world applications. Don’t worry, we’ve got you covered with clear, precise explanations. By the end, you’ll have a solid understanding of isotopes and their role in our universe. So, are you ready to get your science on? Let’s explore!
Understanding Basic Atomic Structure
To fully grasp what isotopes are, you’ll first need to understand the basic structure of an atom. It’s made up of three kinds of particles: protons, neutrons, and electrons. Protons and neutrons form the nucleus at the center of an atom, while electrons orbit the nucleus in specific patterns, known as electron configuration.
The number of protons determines the element’s atomic number, and it’s unique to each element. For example, the element hydrogen has one proton, so its atomic number is one. The sum of protons and neutrons gives the atomic mass of an atom. This is where isotopes come in – isotopes of an element have the same number of protons, but different numbers of neutrons. Hence, they have different atomic masses.
The calculation of atomic mass is not a straightforward count of protons and neutrons. Instead, it’s a weighted average that takes into account the relative abundance of an element’s isotopes. Understanding this process is crucial when you’re dealing with isotopes, as it helps you to make sense of their properties and behavior.
Defining the Term ‘Isotope
So, you’re probably wondering what exactly an isotope is. Well, in the simplest terms, an isotope is a variant of a chemical element that shares the same number of protons but has a different number of neutrons in its nucleus. This difference in neutron count creates isotopes of the same element with varying atomic masses.
Let’s address some isotope misconceptions. Many believe that isotopes are a different element altogether, which isn’t accurate. They’re simply variations of the same element, bearing the same atomic number but differing atomic weights.
The isotope discovery was a significant breakthrough in nuclear chemistry. The credit for this monumental scientific achievement goes to Frederick Soddy. In 1913, he identified the presence of isotopes in a radioactive decay series.
Understanding isotopes is crucial in many scientific fields. For instance, in nuclear physics, they’re used to determine the age of ancient artifacts through radiocarbon dating. In medicine, isotopes play a vital role in diagnostic imaging and cancer treatment.
Formation of Different Isotopes
With the basics of what isotopes are under your belt, let’s delve into how these different versions of the same element actually form. Isotopes are formed when an atom’s nucleus undergoes change. This change can occur naturally, like in radioactive decay, or it can be induced artificially in nuclear reactors or particle accelerators.
Here’s a brief table to illustrate the formation of three isotopes:
| Isotope | Formation Process | Isotope Stability |
|---|---|---|
| Carbon-12 | Formed in stellar nucleosynthesis | Stable |
| Carbon-14 | Produced in the atmosphere | Unstable |
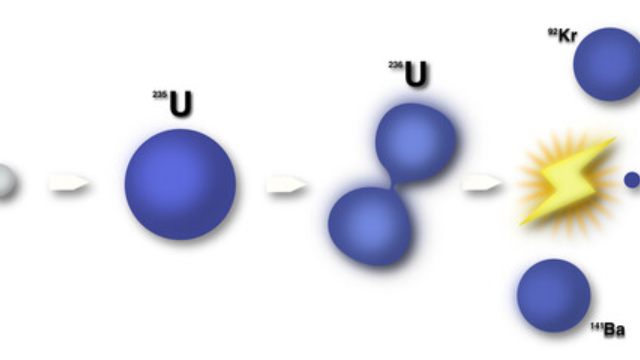
| Uranium-235 | Formed in supernovae | Unstable |
Isotope Applications are varied and numerous. For instance, Carbon-12 is used in carbon dating to determine the age of ancient artifacts, while Uranium-235 is utilized in nuclear reactors for electricity generation.
Isotope Stability is crucial in understanding the behavior of isotopes. Stable isotopes remain unchanged over time while unstable isotopes decay, releasing radiation. This characteristic impacts their applications. For example, the instability of Carbon-14 is what makes it useful in carbon dating, as its predictable decay allows us to calculate the age of organic materials.
Understanding the formation and stability of isotopes enables you to comprehend their various applications in scientific and industrial fields.
Types and Examples of Isotopes
While you’ve learned about the formation and stability of isotopes, it’s now time to delve into the different types of isotopes and some specific examples. Primarily, isotopes are categorized into two types: stable and radioactive. Stable isotopes don’t decay over time, hence, they’ve remained unchanged since their formation. Carbon-12 and Oxygen-16 are examples of stable isotopes that you encounter daily.
Radioactive isotopes, on the other hand, are unstable and undergo radioactive decay. They’re crucial in the field of Isotope Applications, particularly in medicine and archaeology. For instance, Carbon-14, a radioactive isotope, is vital in dating archaeological finds. Another example, Technetium-99m, is extensively used in medical imaging.
The Isotope Discovery has broadened our understanding of the natural world. Each isotope, with its unique set of protons, neutrons, and electrons, exhibits distinct characteristics and behaviors. Understanding these isotopes and their properties isn’t just about comprehending the atomic world. It’s also about applying this knowledge to solve real-world problems. So, as you explore isotopes, remember, you’re not just learning about atoms; you’re unlocking the potential to transform the world.
Isotopes in Everyday Life
In the midst of your daily routine, you’re likely encountering isotopes without even realizing it. Isotopes, variations of elements with different neutron counts, are pervasive in our daily lives. Their applications range from medical diagnostics to smoke detectors, and even in your morning coffee.
Isotope applications are numerous in medicine. For instance, you’ve probably heard of PET scans. These employ fluorine-18, a radioactive isotope, to detect and visualize cancerous cells in your body. Similarly, iodine-131 treats thyroid conditions, while technetium-99m aids in imaging the skeleton and heart muscle.
Isotopes also have a place in your kitchen. Ever wondered why your smoke detector works so efficiently? It contains americium-241, an alpha particle-emitting isotope. When smoke enters the alarm, it interrupts the alpha particles, triggering the alarm.
Furthermore, isotopes make your morning coffee possible. Carbon-14 dating, a method of isotope detection, helps determine the age of ancient plant-based materials, including coffee!